Development of lateral flow device for species
identification of snake bite and subsequent development
of mobile phone based application for payload
quantification
Department
of Science Technology [DST]
Research Summary
LMMD is a diagnostic support arm for the public, fulfilling the social relevance of RGCB. LMMD has taken initial steps to pervasive its diagnostic services in and around Kerala. LMMD is the only lab of its kind in India that performs more than 50 parameters under one roof .The lab knobs both diagnostics and research equally. The ongoing research projects of the lab are with the objective of providing social benefit. Currently LMMD has two ongoing projects,
- Development of lateral flow device for species identification of snake bite and subsequent development of portable reader for payload quantification.
- Formulation of a predictive marker of drug metabolism through a pharmacogenomics approach to alleviate Tacrolimus-induced undesirable effects on transplant recipient.
Research Programs

This project mainly focuses on the identification of snake species with the help of a Point of care device as well as quantification of payload using mobile phone based application.
India is the second most populous country in the world with over 1.3 billion people .Every year around 8.3 million people die in India. According to WHO India has the highest Snake bite mortality in the world. Nearly 49000 death cases are reported in India each year. Many
Unreported cases are also there. Mainly four snakes species are primarily responsible for the reported cases of highest death rate. The big four species are Common Cobra (Najanaja), Krait (Bungaruscaeruleus), Russells Viper (Daboiarusseli ) and Saw Scaled Viper (Echiscarinatus).The major cause of death is due to the failure in identifying the snake species and in lacking suitable technology for payload quantification . Once the snake species and payload amount is known at the initial stage of snake bite, it’s easy for the clinicians to provide effective treatment .This project mainly focuses on the identification of snake species with the help of a Point of care device as well as quantification of payload using mobile phone based application.
Point of care (POC) testing has become the most well-known way of diagnosis in clinical analysis, food safety and environment. Lateral flow Assay (LFA) based POC devices are among very rapidly growing approach for qualitative as well as quantitative analysis. Compared to other techniques ( eg: Enzyme Linked Immunosorbent assay(ELISA)) , POC provide quick results in shorter times. Rapidity, one step analysis, low operational cost, simple instrumentation, user friendly format ,high sensitivity and specificity ,long life span and portability of the device are the exceptional qualities of LFA strip. The present investigation is aimed to develop a lateral flow device to identify snake species and subsequent development of mobile phone based application for payload quantification
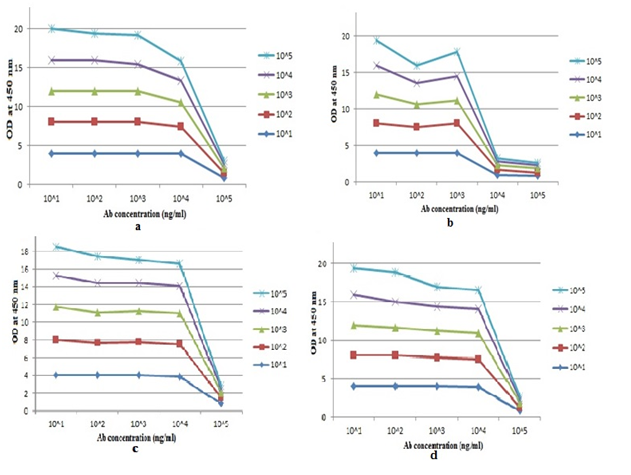
Fig: 1. Study by ELISA: Each of the four venoms was
coated on a micro- ELISA plate then tested with
polyvalent venom antisera.The result shows OD value of
four antisera against four venom.(a) Indian Cobra (Najanaja),
common krait (Bungaruscaeruleus),
Russell’s viper (Dabiorusselii) and
saw-scaled viper (Echiscarinatus).

Fig:2. Validated
protocol of lateral flow assay

Transplantation is typically the standard of therapy for all patients with end-stage liver or kidney disease. Since the success of the transplant depends on a delicate balance between immunosuppression and rejection, reaching and maintaining an adequate therapeutic level by giving appropriate doses of immunosuppressive drugs is extremely important, especially in the first phases after the transplant.
Optimizing balance between therapeutic efficacy and occurrence of adverse drug reactions is critical in narrow therapeutic drugs like Tacrolimus (Tac), an immunosuppressive drug used to prevent solid organ rejection. Tac requires therapeutic monitoring of blood concentrations to prevent graft rejection owing to inadequate immunosuppression with low drug levels or toxicity due to high drug levels. It exhibits inter-individual pharmacokinetic variability, which affects the dose required to reach the target concentration in blood. Even with much progress in medicine, the fragile equilibrium between the risks and benefits of immunosuppression makes the management of immunosuppressive pharmacotherapy a challenge.
The main objective of the project is to develop a strong predictable marker with clinical applicability specific to our population, which can predict an individual’s response to Tac, so that patients at high risk of developing Tac-related complications can be identified even before their organ transplant. Though many studies largely concentrating on the role of CYP3A5 on Tac blood levels have been carried out, the pharmacogenetic factors identified so far are not of sufficient predictive value to be of much clinical use. Also, the lack of consensus about the role of CYP3A5 in the pharmacokinetics of Tac hinders the development of assays with clinical applicability.
Tac is metabolized by CYP3A in the gut and liver, and transported in the gut by P-glycoprotein (P-gp), an efflux pump, which is encoded by the multidrug-resistant protein (MDR1)/ABCB1 gene. Thus, CYP3A5, CYP3A4 and ABCB1 polymorphisms could have an important role in dose requirements.
We propose to investigate the role of polymorphisms in these three important genes and their interaction in influencing Tac response and dosing strategies in organ transplant recipients in South Indian population. Patients undergoing renal transplantation will be recruited from hospitals in Trivandrum, Kerala. DNA isolated from peripheral blood will be used to screen polymorphisms in CYP3A4, CYP3A5 and ABCB1 genes and correlated to the Tac blood levels in patients. Gene-gene interactions will be analysed to develop a better predictability marker involving a combination of functional variants.
Current Research Grants
-
2019 2016
-
2018 2016
Formulation of a predictive marker of drug metabolism through a pharmacogenomics approach to alleviate Tacrolimus-induced undesirable effects on transplant recipients.
SERB, Department of Science & Technology [DST]
Research Grants Completed
-
ICMR Virology Network Program 2011-2016
