Deciphering the regulation of PiwiL1 in Cancer Stem cells
Science and Engineering Research Board [DST-SERB]
Research Summary
The main focus of my research is to understand the involvement of non-coding RNAs and associated proteins in the stem cell maintenance in normal as well as cancer conditions. My research also involves elucidation of epigenetic mechanism behind the regulation of multidrug resistance proteins in embryonic carcinoma stem cells.
Research Programs
Retinal degeneration that involves photoreceptors is the cause for Age related macular degeneration (ARMD) and Retinitis Pigmentosa (RP). Cell replacement therapy is the only feasible treatment for such diseases. Efforts have taken to generate photoreceptors from various sources, specifically from hES cells. Stem cell therapy includes three major pre-requisites. Current research is focused on the fine tuning of internal cell machinery to increase the efficiency of photoreceptor generation. Recently, miRNAs have been shown significant involvement in various cellular functions. miRNAs are small RNA molecules of 18-22 nucleotides in length which regulate expression of various genes. We found that miR cluster 143/145 directly binds and inhibits Nrl and over-expression of this cluster reduces the photoreceptors in mouse retina. Temporal analysis revealed a differential expression pattern for these miRNAs in different stages of retinal development in mouse retina. They are down-regulated during photoreceptor development suggesting an in vivo mechanism that regulates their expression for the generation of rod photoreceptors. We also observed an autoregulatory mechanism in which Nrl positively regulates miR cluster 143/145 and this autoregulation may be needed for maintaining the rate of photoreceptor generation. Understanding the specific miRNAs in the photoreceptor development is crucial, since manipulation of their expression could potentially be used as a therapeutic tool for treating photoreceptor degenerative diseases in future.
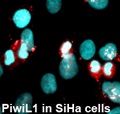
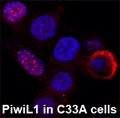
One of the major causes for cancers is epigenetic regulation at the chromatin level and any changes in epigenetic regulators such as mutations or alterations can lead to tumorigenesis. Balance of chromatin remodeling activities and epigenetic regulators has to be maintained in order to prevent the transformation of a normal cell into a cancerous cell. Cancer-associated epigenetic changes are achieved via DNA methylation, covalent modification of histones, and changes in the association of epigenetic factors with chromatin. However, most epigenetic factors, such as DNA methyltransferases/demethylases and histone modification enzymes, do not recognize specific DNA sequences. Recent studies indicate that Piwi proteins and associated RNAs can recruit epigenetic factors to their target sites. It is important to understand whether the epigenetic changes in cancer are mediated by Piwi proteins or not. The observations that the Piwi proteins are aberrantly expressed in a variety of cancers and play significant roles in proliferation, apoptosis and cell invasion/metastasis, suggest a possibility for involvement of Piwi proteins in the epigenetic variations in cancers. In spite of the growing attention focused on Piwi proteins, only a few studies have been done to understand the molecular mechanisms by which Piwi proteins contribute to tumorigenesis or function in cancer cells. Here, in this study, we are trying to identify the regulatory RNAs that are interacting with Piwi proteins and also their functions in HPV-associated epithelial cancers. Understanding the role of Piwi proteins in HPV-associated epithelial cancers and how they are regulated in cancers in response to HPV will have an impact at the clinical level since it allow to formulate a better diagnostic as well as therapeutic approaches to address such cancers.
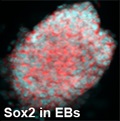
Germ cell tumors (GCTs) are malignant or non-malignant tumors which represent a histologically heterogeneous group of neoplasms derived from the germ-cell lineage. Despite their heterogeneity, they are all presumed to arise from totipotent primordial germ cells (PGC). Most of the ovarian and testicular cancers are of germ cell origin. In western countries, testicular GCTs (TGCT) account for up to 60% of all malignancies diagnosed in male patients between 20 and 40 years of age. Histologically, GCTs can be divided into germinomas and non-germinomas. Germinomas (GERs; also called seminomas in the testis and dysgerminomas in the ovary) are tumors of undifferentiated germ cells that retain markers of pluripotency. In contrast, non-germinomas undergo differentiation to resemble somatic-type tissues (teratomas) or extra-embryonic structures (yolk sac tumor (YST) and choriocarcinoma. Nonseminomas contain one or more histological subtypes representing various differentiation lineages and stages of embryonic development. Embryonal carcinoma cells represent the stem cell component which has the potential to differentiate towards embryonic and extra-embryonic tissues. Though GCTs are rare, they account for about 2-4% of pediatric cancers and cancers occur in adolescents younger than age 20. These tumors can spread to the other parts of body such as lungs, liver, lymph nodes, central nervous system. Usually GCTs are highly sensitive to chemotherapy and 80% cure can be achieved with cisplatin (CDDP)-based combination chemotherapy followed by secondary resection in the case of residual tumor lesions. But mature teratomas, despite an identical genetic constitution, do not share the general chemosensitivity of GCTs. Due to intrinsic chemotherapy resistance, mature teratomas can be found in ~30-40% of residual lesions after chemotherapy. 10-30% of patients with metastatic GCT were unable to achieve a durable complete remission after initial treatment, either due to incomplete response or relapse. Though most of the GCTs are sensitive to chemotherapy, malignant differentiated GCTs such as TGCTs display higher resistance to chemotherapy. Based on this clinical background, the understanding of the mechanisms of chemosensitivity and resistance of tumor cells is becoming more important in order to further improve therapeutic outcome. A more accurate prediction of treatment outcome may help to avoid under- or overtreatment. Here, in the proposed study, we are trying to elucidate the mechanism of regulation of multidrug resistant proteins in pluripotent stem cells. As the tools that influence specific cellular pathways are evolving, they may allow individual resistance mechanisms to be reversed or overcome, which might help to cure more patients in the future.
Current Research Grants
-
2024 2021
Previous/ Completed Research Grants
-
DST SERB Fast Track Grant
Department of Science & Technology [DST] 2010-2013CSIR EMR grant
Council of Scientific and Industrial Research [CSIR] 2017-2020DBT EMR grant
Department of Biotechnology [DBT] 2018-2020
